
Contact us
Our team would love to hear from you.
A single secure environment for collaboration of medical specialists during clinical trials of new drugs and medical devices.

Sentral Clinical Research Services, LLC (SCRS) is a U.S. company specializing in medical research of new drugs and medical devices. The company’s aim is to facilitate the participation of physicians and patients in clinical research.
A clinical trial is a complex process that involves a lot of documentation and cooperation of many medical specialists both at dedicated research facilities and in the private practice setting. Physicians’ collaboration and medical data analysis constitute an important part of the process.
As the amount of research data expanded, the company reached out to EffectiveSoft to develop a unified document management system that would be highly flexible and user-friendly, keeping document version control and approval workflows.

The customer had only a general idea of what the system should look like. During the regular meetings our team discussed the system wireframes and functional specifications with the client to ensure that all the customer’s requirements were met.
As a result, our engineers developed the document management system SentralBinders. It simplifies teamwork, expedites document exchange (in Microsoft Word and Adobe PDF formats), and ensures mobility of participants due to access available from different devices (both PCs and iPads).
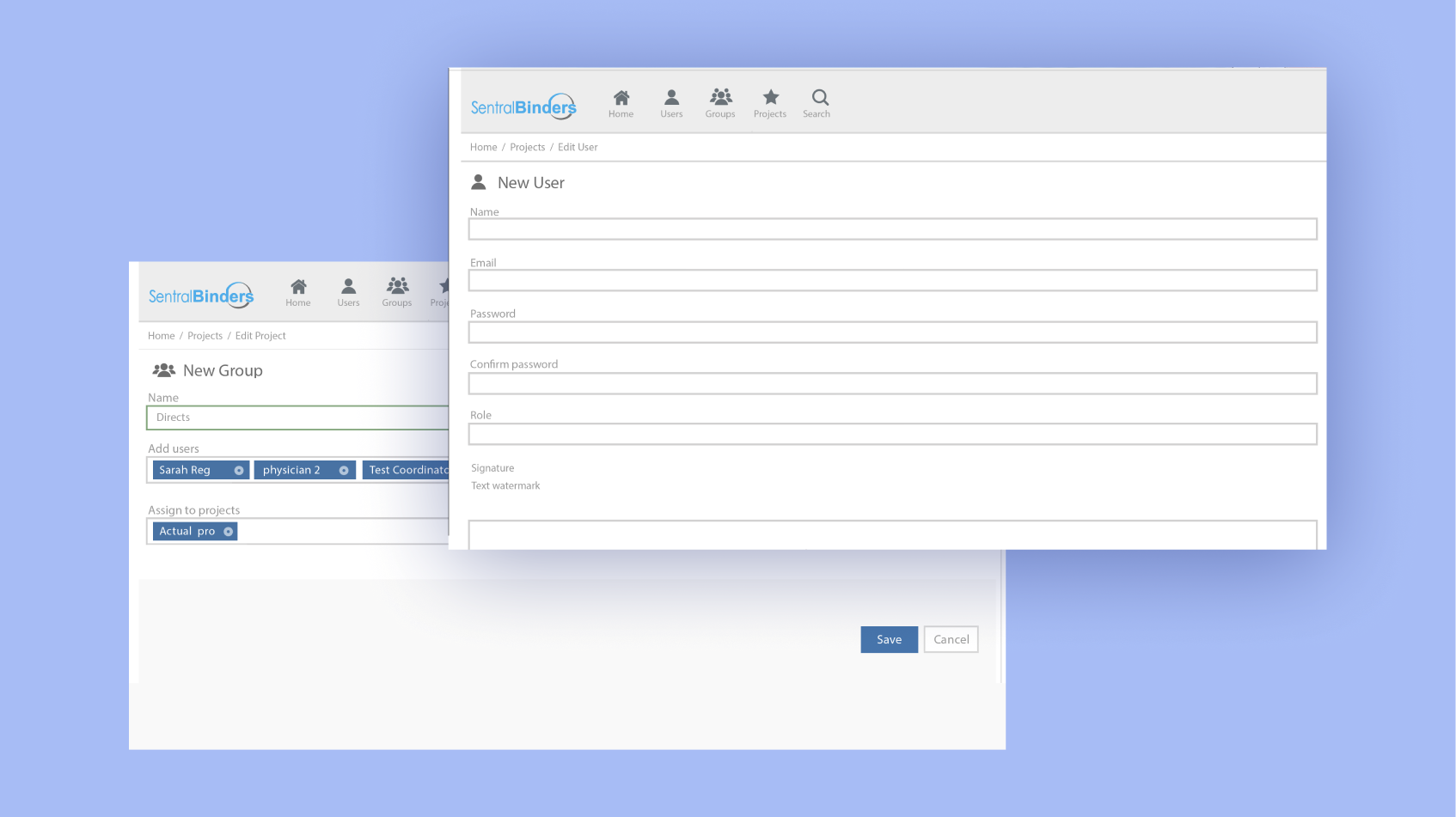
Transparent and automated workflow
Flexible and reliable data management
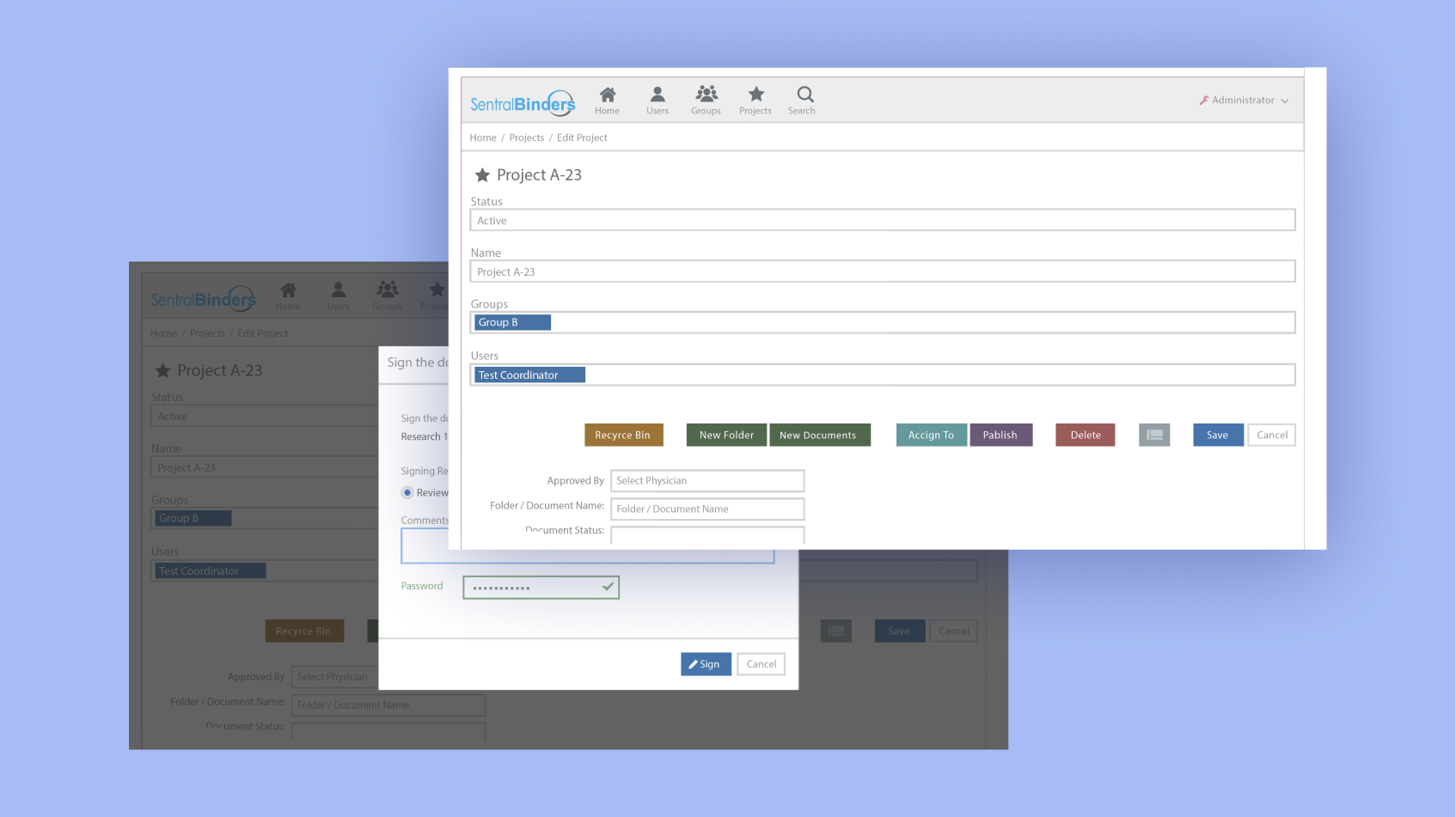
Simplified version control and history tracking
Our team would love to hear from you.
Fill out the form to receive a free consultation and explore how we can assist you and your business.
What happens next?